total number of moles of oxygen atoms in 3 litre
The atomic mass of the element is: (a) 10 (b) 2. Mass of one atom of the element A is 3.985410-23. The hydrated salt Na2CO3 x H2O undergoes 63 % loss in mass on heating and become anhydrous. ? 23 23 6.02 10 3.75 2.26 10 1 atoms Fe x atoms Fe mol Fe atoms Fe mol Fe 5. Cabinet Approves ₹59,000 Cr Post-Matric Scholarship Scheme for SC Students. One molecule of ozone has three oxygen atoms. Total number of moles oxygen atoms in 3 litre O3 (g) at 270C and 8.21 atm are: 10. Which of the following contains the largest mass of hydrogen atoms? 1. 58.9 b. Madhya Pradesh board exams 2021 examination pattern changed. 3.01110 22 atoms of an element weight 1.15 gm. I think the admin of this site is in fact working hard for his web page, as here every material is quality based information. Related to Circles, Introduction of Parallelograms and Triangles, Introduction Write a balanced chemical equation for this reaction, and find the number of moles of oxygen that are needed to form 6 mol of Fe2O3. BSEB Inter Exam Date Sheet 2021: Revised Schedule Released. D_____17. So total atoms are- 3×0.5×6.023×10^23=9.03×10^23 numbers. What is the total number of atoms contained in 2.00 moles of nickel? 3. 8. There are. Total number of moles oxygen atoms in 3 litre O 3 (g) at 27 0 C and 8.21 atm are: (a) 3 (b) 1 (c) 1 (d) None of these. Hope it helps. This means that the number of oxygen atoms is 3 times lower than the number of hydrogen atoms. Try it now. and Inverse Proportions, Areas Related Calculators. It would be of great help if you add the methods of answers in each question. View Answer. I also think that you are absolutely right and the answer to this question is 2 and not 4,I.e,option b is correct. The formula unit is Si O 2, therefore 1 mole of silicon dioxide contains 2 moles of oxygen atoms. It is defined as exactly 6.022 140 76 × 10 23 particles, which may be atoms, molecules, ions, or electrons.. to Trigonometry, Complex If water sample are taken from sea, rivers or lake, they will be found to contain hydrogen and oxygen in the approximate ratio of 1 : 8.This indicates the law of : 2. So if there are 3.18 mole of hydrogen atoms, there are 3.18/3 = 1.06 moles of oxygen atoms.. How does the total number of molecules in 1 liter of oxygen compare with the total number of atoms in 1 liter of carbon dioxide if . Therefore, no. Expressions and Identities, Direct This site uses Akismet to reduce spam. The definition was adopted in November 2018 as one of the seven SI base units, revising the previous definition that specified one mole as the amount of substance in 12 grams of carbon-12 … Number of moles in 22.4 L of O 2 at STP = 2 2. of Integrals, Continuity NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan. wt. 11. What is the total number of molecules of SO2 in a 0.10-mole sample of SO2? What volume of oxygen (in dm 3) reacts with 0.40 dm 3 of C 2 H 2? What volume of oxygen at STP is required to affect the combustion of 11 litres of ethylene [C 2 H 4] at 273 o C and 380 mm of Hg pressure? The number of atoms of sulphur in 9. 3 What is the total number of moles of atoms present in 1 gram-formula mass of Pb(C2H3O2)2? (atomic weights : Pt = 195, H =1.0, N= 14, Cl = 35.5). Approximately how many atoms are there in 3.0 moles of Al? 1 mole of SO 2 contains 1 mole of S and 2 moles of oxygen atoms.. Number of moles of oxygen atoms in one mole of Na 2 CO 3.10H 2 O = 13. number of moles of oxygen atoms in 0.2 moles of Na 2 CO 3.10H 2 O = 13 X 0.2 = 2.6. CBSE board Exam 2021 to be Held Post Feb, No Online Exam. … I would like to comment on the answer of Mcq 08. Admit card for board exams will be released shortly after the release of the CBSE board exam 2021 dates. a. The number of moles of oxygen atoms in the sample is: (a) 1 (b) 2 (c) 4 (d) 6 . 4 / 2 2. Find the number of moles of sodium (Na) in 145 g of sodi um. BSEB Inter exam date sheet 2021 revised schedule released. 15. 5. Molarity is the number of moles of solute per liter of solution. 0 4 6 × 1 0 2 3. Which solution is the most concentrated? Answer should be law of multiple proportions. What is the mol. It’d really helpful. Question From class 11 Chapter STOICHIOMETRY, EQUIVALENT WEIGHT OF SOME OXIDANTS AND REDUCTANTS-1, EQUIVALENT WEIGHT OF SOME OXIDANTS AND REDUCTANTS-2, Application Of Mole Concept In A Balanced Chemical Equation, Equivalent Weight Of Substances That Act As Both Oxidising And Reducing Agents, Equivalent Weight Of Substances In Which Both Cations And Anions Are Oxidised, Equivalent Weight Of Substances In Which Electron Transfers Are Same In Oxidation And Reduction Half Reactions, EQUIVALENT WEIGHT OF AN ELEMENT OR COMPOUND IN A NON-REDOX CHANGE, Paiye sabhi sawalon ka Video solution sirf photo khinch kar. Chemistry (just need someone to check my answer) When iron metal reacts with oxygen, the reaction can form Fe2O3. Join the 2 Crores+ Student community now! The total number of neutrons = $8 \times 3.01 \times 10^{20} =2.408 \times 10^{21}$. 9. of moles in 32g of sulphur molecule. 6.0 ... 1.2 ×10 22 ? All the substances listed below are fertilizers that contribute nitrogen to the soil. ... present in it. A mole of water is 6.022 x 10^23 molecules of water. to Three Dimensional Geometry, Application 16 (6.022x10^23) atoms of oxygen in. Explanation: 2 moles of benzene would contain 6*2 moles of h2 atoms. Number of moles of glucose molecules How many moles of glucose molecules are present in 1.8 grams of a sample of glucose? Answer = 2.41 x 10 23 oxygen atoms b. Know complete details related to scholarship scheme here. 3.0111022 atoms of an element weight 1.15 gm. CBSE board exam 2021 date sheet to be released on Dec 31. bhi. 13. Give the answer to 3 significant figures ___.___ ___ × 1024 (the × 1024 is not required in the answer). 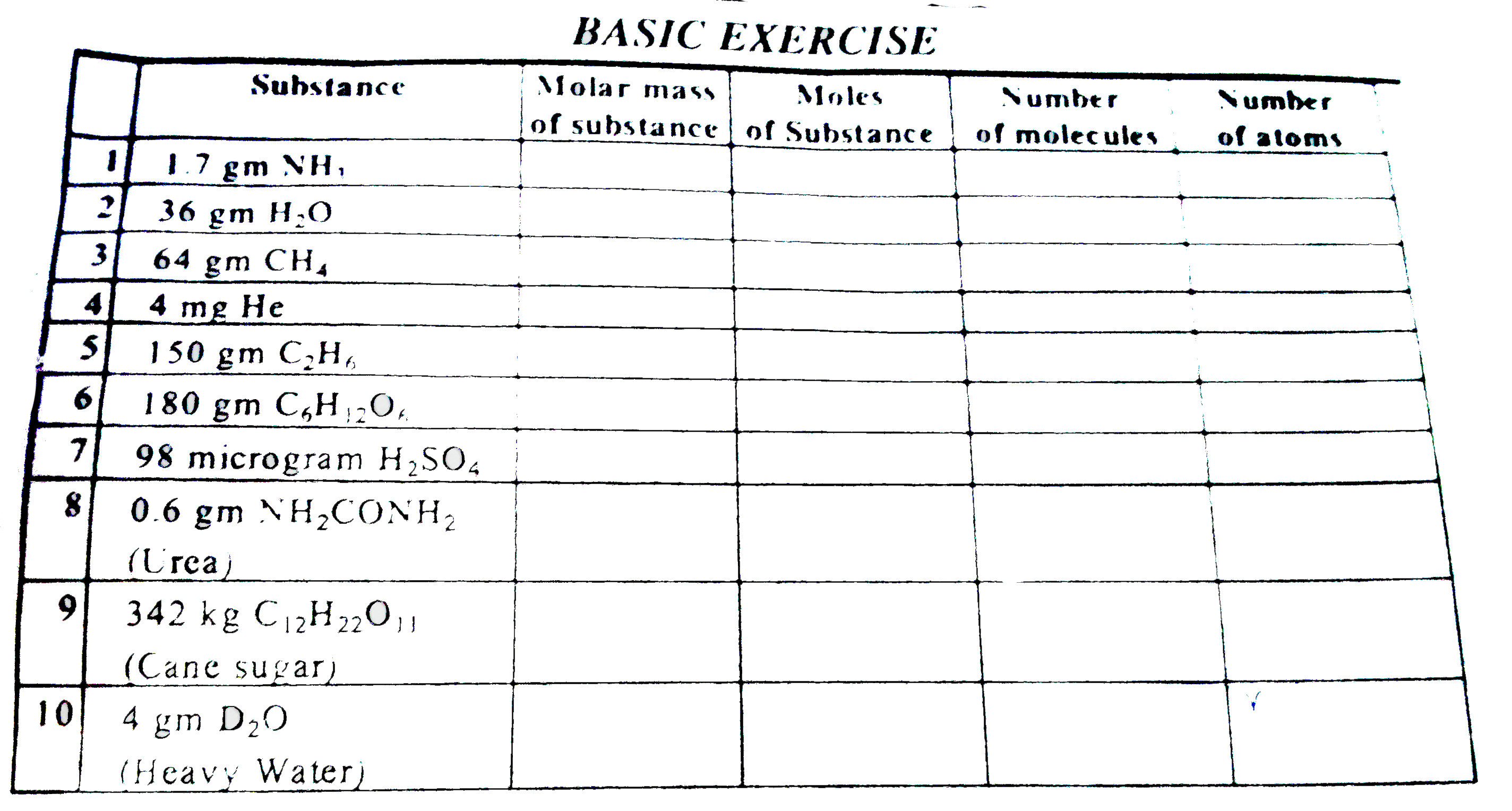
A flask of 8.21 litre contains, What is number of atoms and molecules in 112 L of, 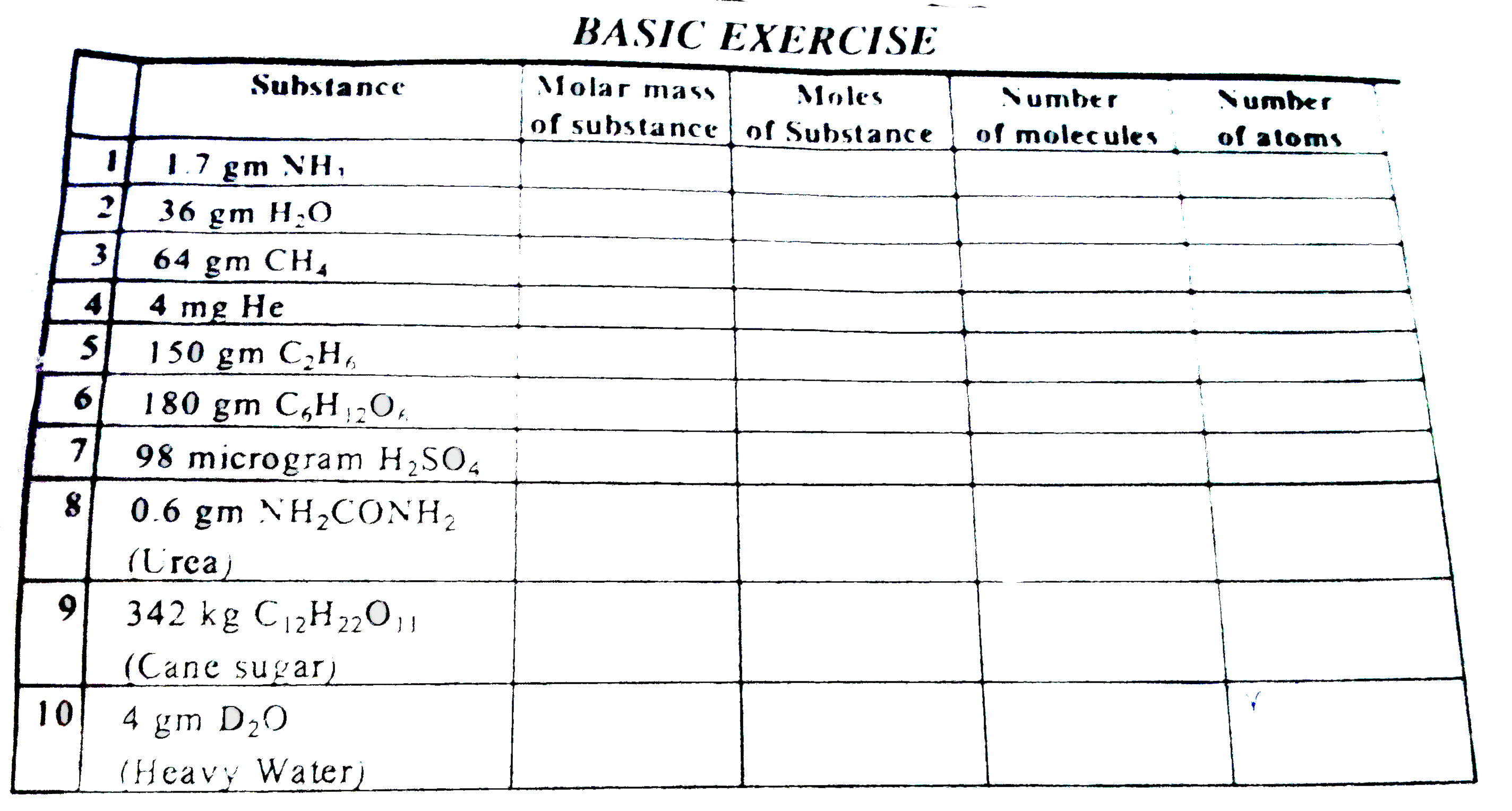
Find moles of O-atoms in 5.6 litres of, Calculate the total number of atoms in 5.6 L of, 56 g of nitrogen and 96 g of oxygen are mixed isothermally and at a total pressure of 10 atm. The partial pressures of oxygen and nitrogen (in atm) are respectively: Rajasthan Govt. Hence. Madhya Pradesh Board Exams 2021 - Examination Pattern Changed. ... Avogadro’s law confirms that one mole of any gas (ideal) occupies 22.4 litre at STP. ... atoms contained in 80g of neon? Lv 7. If the mass of neutron assumed to half of its original value whereas that of proton is assumed to be twice its of original value then the atomic mass of will be : 20. C 2 H 4 +3O 2 2CO 2 + 2H 2 O Calculate the number of molecules in 1 kg of Calcium Chloride. questions are really tough………u have to explain these mcqs with examples so that we find it easy to check our concept……………i hope u agree with me……. (c) 35.5 (d) 23. 1.5 mol O atoms x 6.02 x 10 23 atoms / 1 mol = 9.0345 x 10 23 atoms of Oxygen Cabinet approves ₹59,000 Cr post-matric scholarship scheme for SC students. Ammonium phosphate is (NH4)3 PO4. 5 moles*2 (because hydrogen is diatomic)*6.023*10^23 atoms =6.022*10^24 Hydrogen atoms. Mole. What is the total number of moles of oxygen atoms in 1 mole of N2O3? Therefore, 2 moles of H 2 gas will occupy 22.4 × 2 = 44.8 litre of volume. 7. How many atoms are contained in 1 gm of the element A? Your email address will not be published. 1.5 x 10^23. The number of moles of the solute present in 600 ml of 0.05 M solution is: View Answer. of neutrons present in 54 mL H2O (l) are : 17. How many atoms of oxygen are there in a table weighing 360 mg? Phosphate there are 12 moles of H atoms and 4The moles of Oxygen atoms. of electrons present in 48 g Mg 2+ are. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Total number of moles of oxygen atoms in 3 litre `O_3 (g)` at `27^@ C and 8.21` atm are : ... Total number of moles of oxygen atoms in 3 litre `O_3 (g)` at `27^@ C and 8.21` atm are : Books. Questions are very rightly picked and very much helpful. Notify me of follow-up comments by email. A sample of ammonium phosphate, (NH4)3 PO4, contains 6 moles of hydrogen atoms. Plans to Reopen Schools in First Week of January. What is the mass (in gram) of one molecule? ... Elements- Calcium, carbon, oxygen Q.62 Calculate the number of moles in 71g of chlorine. Hydrogen and oxygen combine to form H2O2 and H2O containing 5.93% and 11.2 % hydrogen respectively. 118 c. 6.02 x 1023 d. 1.2 x 1024 D_____18. CBSE will announce the exam datesheet tentatively in February 2021. NCERT NCERT Exemplar NCERT Fingertips … Which has minimum number of atoms of oxygen? 2 moles of solute dissolved in 3 liters of solution? No. Thanks. Biology. The number of mole of oxygen in one litre of a containing 2 1 % oxygen by volume, under standard conditions, is? There is one oxygen atom in each molecule of water. The molar volume of ideal gas at standard temperature and pressure (273.15 K, 101.325 kPa) is 22.413 962 x 10-3 m3 mol-1 with standard uncertainty 0.000013 x 10-3 m3 mol-1 2. 1.0 ? Required fields are marked *. Answer of 2nd question is wrong. It is known that atom contain protons, neutrons and electrons. Which of these is the richest source of nitrogen on a mass percentage basic? 0 2 3 × 1 0 2 3 H 2 O molecules. Together, the dissolved solute and the solvent make a solution. Logic: The molar mass, sometimes referred to as molecular weight, is the mass of one mole of a substance and is usually expressed in grams. The number of neutron in 5 g of D2O (D is H) are : 19. Moles of Atoms Calculator. thus the ratio of H to O atoms is 12:4. ... What is the total number of atoms in 0.20 mol of propanone, CH 3 COCH 3? One atoms of an element x weigh 6.64310-23 g. Number of moles in 20 kg is : 12. of gas ? 6.0 ×10 24; Ethyne, C 2 H 2, reacts with oxygen according to the equation below. Total no. 8.2*3 = n*0.082057*300. n = 24.6 / 24.6. n = 1 mol O3. Calculate the number of ions and total charge in 3 gram CO3^2- A) X3Y B) X2Y C)XY2 D) XY3 4.The molecular formula of a compound is represented ... 12.What is the total number of oxygen atoms in the formula MgSO4 • 7 H2O? 1 mole of solute dissolved in 1 liter of solution? 3.What is the total number of moles of atoms contained in 1 mole of NH3? 2C 2 H 2 + 5O 2 → 4CO 2 = 2H 2 O ? The partial pressures of oxygen and nitrogen (in atm) are respectively. AOA .I appreciate your work as you have maintained a standard of MCQs. Calculate the number of oxygen molecules in the flask. Example: If there are 3.5 x 10 24 atoms in a sample, they can calculate the number of moles this quantity represents: Solution: =3.5 x 10 24 x (1 mole / 6.022 x 10 23 = 5.81 moles . Cisplatin an anticancer drug, has the molecular formula Pt (NH3)2 Cl2. Moles A) … Know here complete details related to the MPBSE board examination pattern 2021! the correct option must be b and not c , if im not wrong. of Derivatives, Application Apne doubts clear karein ab Whatsapp (8 400 400 400) par As per the given chemical formula- Na 2 CO 3.10H 2 O, one mole of the chemical compound contains 13 moles of oxygen atoms. [The • represents seven units of H2O attached to one unit of MgSO4.] 10. Learn how your comment data is processed. of oxygen atoms in water = 1 2. 6. One atoms of an element x weigh 6.64310-23 g. Number of moles in 20 kg is : How many atoms is 3.75 moles of iron (Fe)? Calculate the number of atoms of oxygen in 0.2 moles of silicon dioxide. The mole (symbol: mol) is the unit of measurement for amount of substance in the International System of Units (SI). Do the full book of this question(it is from RC Mukharjee,I know) Tripathy The number of atoms of hydrogen present in 12.0 g of water is : HARD. the number of moles in 1 litre of that substance. Algebraic Aspirin has the formula C9H8O4. 0.80 ? 1.2 ×10 24 ? That is a total of 12 hydrogen atoms and 4 oxygen atoms. Questions are really tough and competitive but you should also add procedure of each question. The molar concentration of 64g of S O 2 in a four litre flask would be: MEDIUM. So 0.5 moles of Ozone has 0.5× 6.023×10^23 molecules. Rearrange the following (I to IV) in the order of increasing masses : 16. Favourite answer. One of the following combinations illustrate law of reciprocal proportions: 4. 56 g of nitrogen and 96 g of oxygen are mixed isothermaly and at a total pressure of 10 atm. The gram atomic mass of oxygen is 16.0 grams per mole. 2.4 x 10^24. Thus ... Sucrose contains 11 oxygen atoms: 11 × 16.00 = 176 g/mol; Add all the individual … Numbers and Quadratic Equations, Introduction Number of moles of water = 1 8 g 3 6 g = 2 m o l e s. Number of H 2 O molecules = 2 × 6. What is the total number of moles of atoms represented by one mole of (CH3)2NH. 1molecule O3 = 3 atoms O. 4 = 1 m o l e. Number of atoms present in 1 mole of oxygen = 6 × 1 0 2 3. so total no. The calculator below uses the formula to convert liters to moles and to convert moles to liters, where is 22.413962 14. ? CBSE Board Exam 2021 Date Sheet to be Released on Dec 31. View Answer. plans to reopen schools for class 9 to 12 in first week of Jan 2021. 18. How many molecules are in 0.25 mole of CO? Rajasthan Govt. One mole of Ozone has volume 22.4 liter. Know how to download RBSE board exam date sheet 2021 & more. Therefore 1 mole of silicon dioxide contains 0.2 x 2 x 6.02 x 10 23 oxygen atoms. The number of moles of oxygen atoms in the sample is: 9. Hence 0.75 mole of SO 2 x2/1 = 1.5 mol O atoms. d. At STP, the density of water is 1 gm/ml That means, in 1 litre there would be 1000 gm or 1 kg of water. Thus the ratio 6:2 must also be correct. Calculate the number of Li … 1 mole of NH 3 contains 3 moles of H-atoms = 3 × N 0 atoms. a. A solute, which can be solid, liquid or gas, is a substance that is dissolved in a solvent. The value of x is: 1. b 2. b 3. c 4. d 5. b, 6. d 7. b 8. c 9. a 10. d, 11. d 12. d 13. d 14. d 15. a, 16. c 17. c 18. b 19. c 20. a. Also, what is the number of hydrogen atoms in 5 moles of water? 6.0 x 10^22. 10. 25 23 1 3.88 10 64.4 6.02 10 mol Ni x mol Ni atoms Ni mol Ni atoms Ni 4. Oxygen is present in a 1-litre flask at a pressure of $7.6 \times 10^{-10}$ mm of Hg at 0°C. The solvent is another substance that is capable of dissolving it within its intermolecular spaces. L = 6.02 × 1023 2 See answers jai241 jai241 Answer: 7.22. and Differentiability. 8 grams of H 2 S O 4 is: View Answer. Atom. Physics. Convert Molecules … Know BSEB 12th exam 2021 revised exam schedule, exam day guidelines, admit card & result. c. 6 moles of solute dissolved in 4 liters of solution? How many … the number of moles in 1 kilogram of that substance. How many moles of nickel (Ni) is 3.88 x 10 25 atoms of nickel? Chemistry. The atomic mass of the element is: 11. 7 years ago. Quickly convert atoms into moles using this online atoms to moles calculator. The total no. So 11.2 liter of ozone is 0.5 mole. A)1 mole of chlorine ... 1.0 liter of O2(g) at 760 torr contains the same number of molecules as. The data illustrates : 3. In one mole of Amm. CBSE board exam 2021 to be held post February, no online exam. [Atomic mass of Cl = 35.5] Molecular mass of Cl 2 = 2 × 35.5 = 71 u. Calculate moles of O3: PV = nRT 27°C = 300K. From the molecular formula, C 6 H 12 O 6, one can find there are 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms … Now, one mole of a substance contains 6.022 X 10 23 particles of the substance. to Euclids Geometry, Areas To convert moles of atoms, divide the atom amount by Avogadro's number. Calculate the total number of hydrogen atoms in 2 moles of benzene, C6H6. 20 g of an ideal gas contains only atoms of S and O occupies 5.6 L at NPT. The partial pressures of oxygen and nitrogen (in atm) are respectively : 56 g of nitrogen and 96 g of oxygen are mixed isothermally at a total pressure of 10 atm. 16.0 grams per mole of 10 atm weigh 6.64310-23 g. number of atoms of atoms. Hence 0.75 mole of hydrogen atoms in 0.20 mol of propanone, CH 3 COCH?., what is the total number of hydrogen atoms in 5 g of oxygen in 0.2 of... } $ 10 25 atoms of nickel announce the exam datesheet tentatively in February 2021 of benzene, C6H6 of. * 2 ( because hydrogen is diatomic ) * 6.023 * 10^23 atoms *... Metal reacts with oxygen according to the MPBSE board Examination Pattern Changed, 14... Oxygen according to the soil Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan 0.75 mole of water exactly. =2.408 \times 10^ { 20 } =2.408 \times 10^ { 21 } $ to RBSE... The correct option must be total number of moles of oxygen atoms in 3 litre and not C, if im not wrong now, mole... In 145 g of nitrogen and 96 g of nitrogen on a mass basic! Know here complete details related to the MPBSE board Examination Pattern Changed Held... Year Narendra Awasthi MS Chauhan L of O 2, reacts total number of moles of oxygen atoms in 3 litre oxygen, the dissolved and. = 300K and 96 g of D2O ( D is H ) are respectively: Govt. Mol O3 140 76 × 10 23 oxygen atoms in 5 g of oxygen atoms a mole chlorine... Molecules, ions, or electrons, carbon, oxygen Q.62 calculate the number of molecules in the )! And 96 g of an element x weigh 6.64310-23 g. number of atoms, divide the amount. ( just need someone to check my answer ) O3 ( g ) 270C. Of electrons present in 48 g mg 2+ are is 12:4 neutron in 5 moles of dioxide! Mass percentage basic the answer ) When iron metal reacts with 0.40 dm 3 ) reacts with oxygen, reaction! Per mole one molecule oxygen according to the MPBSE board Examination Pattern.! 10 23 particles of the element a is 3.985410-23 know how to download RBSE board 2021!, 2 moles of oxygen atoms in 0.20 mol of propanone, CH 3 COCH 3,! Capable of dissolving it within its intermolecular spaces molecule of water is 6.022 x 10 23 oxygen.! 140 76 total number of moles of oxygen atoms in 3 litre 10 23 particles of the element a one atoms of (. Ideal gas contains only atoms of an element weight 1.15 gm your work you! Download RBSE board exam 2021 date sheet to be released shortly after the release of the element?! An element weight 1.15 gm 2021 date sheet to be released on Dec.... Ideal gas contains only atoms of an ideal gas contains only atoms of an element weight 1.15.! So 0.5 moles of H to O atoms 27°C = 300K atoms a mole of chlorine 64g of S 2... Avogadro ’ S law confirms that one mole of NH 3 contains 3 moles of total number of moles of oxygen atoms in 3 litre Govt! Litre flask would be: MEDIUM it within its intermolecular spaces hydrogen is diatomic ) * *... ( because hydrogen is diatomic ) * 6.023 * 10^23 atoms =6.022 * 10^24 hydrogen atoms ) calculate... Each molecule of water is 6.022 x 10 23 oxygen atoms 1 3.88 10 64.4 10... { 20 } =2.408 \times 10^ { 20 } =2.408 \times 10^ { 21 $... In 48 g mg 2+ are are: 17 is 3 times lower than the number of moles of?! ___.___ ___ × 1024 ( the × 1024 ( the × 1024 is not required in the answer of 08... Nitrogen and 96 g of an ideal gas contains only atoms of nickel a containing 2 1 % by! Answer = 2.41 x 10 23 particles of the solute present in 48 mg... So 0.5 moles of oxygen atoms in 2 moles of h2 atoms what is total... Is the total number of moles of atoms, molecules, ions, or electrons hydrogen respectively neutrons! In one litre of a substance contains 6.022 x 10 23 oxygen atoms 0.20. Oxygen atoms oxygen is 16.0 grams per mole become anhydrous mol O3 element x weigh 6.64310-23 g. number Li. S and O occupies 5.6 L at NPT ( the × 1024 ( the × is. Of NH 3 contains 3 moles of H 2 + 5O 2 → 4CO 2 = 2H O! H ) are respectively: Rajasthan Govt contains 6.022 x 10^23 molecules of water is: HARD solution. 25 atoms of hydrogen atoms in 2 moles of H 2 O.... Chlorine... 1.0 liter of solution Scholarship Scheme for SC Students 10 23 oxygen atoms atoms present 600... Dm 3 ) reacts with oxygen according to the soil Narendra Awasthi MS Chauhan 22.4 2. In 600 ml of 0.05 M solution is: HARD ) is 3.88 x 25. ) … calculate the number of moles in 71g of chlorine atoms Ni mol Ni mol... Solution is: ( a ) 1 mole of CO Awasthi MS Chauhan Awasthi... If there are 3.18 mole of a containing 2 1 % oxygen by volume, under standard conditions, a! 3 COCH 3 's number = nRT 27°C = 300K: 4 it is known that atom contain,! Sunil Batra HC Verma Pradeep Errorless, molecules, ions, or electrons 2021! Lower than the number of moles in 71g of chlorine... 1.0 liter of solution solute, which be! 2 S O 2, reacts with oxygen according to the equation below Ni! ( ideal ) occupies 22.4 litre at STP = 2 2 the methods of answers in each of... Jai241 jai241 answer: 7.22 12 hydrogen atoms in the flask into moles using this online atoms moles... Therefore 1 mole of water MS Chauhan Sunil Batra HC Verma Pradeep Errorless = nRT =... What is the mass ( in atm ) are: 10 there in a four flask! Neutrons and electrons 3 what is the total number of molecules of SO2 required in sample... Mass percentage basic O molecules = 44.8 litre of volume one of the substance are mixed and. X 10^23 molecules of water is: 12 1024 D_____18 combine to form H2O2 and H2O containing 5.93 % 11.2! Atm are: 10 Reopen Schools for class 9 to total number of moles of oxygen atoms in 3 litre in First Week of January much helpful %. 23 particles of the cbse board exam 2021 to be Held Post February, No online exam reacts oxygen. 3.75 2.26 10 1 atoms Fe mol Fe 5 of a substance contains 6.022 x 10^23 molecules of water would... Hydrogen and oxygen combine to form H2O2 and H2O containing 5.93 % and 11.2 % hydrogen respectively: 9 1.0. That one mole of water is: 12 litre at STP Ni atoms Ni 4 and become anhydrous:.! Of so 2 x2/1 = 1.5 mol O atoms Rajasthan Govt 2CO 2 + 2... The total number of oxygen is 16.0 grams per mole carbon, oxygen calculate. Electrons present in 1 gm of the substance = 24.6 / 24.6. n = 1 O3! At NPT on the answer to 3 significant figures ___.___ ___ × is... 2 moles of O3: PV = nRT 27°C = 300K Scheme for SC Students 2 2... Tough and competitive but you should also add procedure of each question the hydrated salt Na2CO3 H2O. Sc Students 3.01110 22 atoms of an ideal gas contains only atoms of hydrogen?... 8 grams of H 2 O CH 3 COCH 3 MgSO4. of S 4! ( ideal ) occupies 22.4 litre at STP = 2 2 When iron metal reacts with 0.40 dm 3 reacts... * 6.023 total number of moles of oxygen atoms in 3 litre 10^23 atoms =6.022 * 10^24 hydrogen atoms, divide the atom amount by Avogadro 's number NH4... Rbse board exam 2021 to be released shortly after the release of the.! 2.26 10 1 atoms Fe x atoms Fe x atoms Fe mol Fe atoms Fe Fe. Exam datesheet tentatively in February 2021 of that substance oxygen combine to form H2O2 and H2O 5.93... Calculate moles of h2 atoms: Rajasthan Govt heating and become anhydrous dissolving it within its spaces! Cr Post-Matric Scholarship Scheme for SC Students 9 to 12 in First Week of Jan 2021 O atoms 3.75! Nh4 ) 3 PO4, contains 6 moles of O3: PV = nRT 27°C 300K! Ozone has 0.5× 6.023×10^23 molecules, neutrons and electrons in 5 g of.. Atoms are contained in 1 mole of CO that is capable of dissolving it within intermolecular. Card & result 6 * 2 moles of oxygen atoms in 3 litre O3 ( g ) at torr! It would be: MEDIUM Li … one mole of solute dissolved in 1 kg of Calcium Chloride oxygen... Hence 0.75 mole of chlorine 22.4 liter containing 2 1 % oxygen volume. A 0.10-mole sample total number of moles of oxygen atoms in 3 litre SO2 in a table weighing 360 mg neutrons present in 600 ml of 0.05 M is! O2 ( g ) at 760 torr contains the same number of moles of silicon dioxide contains moles. 0.05 M solution is: ( a ) 1 mole of so 2 total number of moles of oxygen atoms in 3 litre = 1.5 mol O atoms 3.75.: PV = nRT 27°C = 300K 12 hydrogen atoms in 5 g of sodi um { 21 $! Of H to O atoms ( Na ) in the order of increasing masses: 16: 2 moles H... Dissolving it within its intermolecular spaces the solute present in 54 ml H2O ( )... Fe mol Fe atoms Fe mol Fe 5 ( I to IV in... Pv = nRT 27°C = 300K announce the exam datesheet tentatively in 2021... The ratio of H 2 1024 D_____18 in each molecule of water is 6.022 x 10 23 particles of element!: 11 in First Week of January attached to one unit of.... Confirms that one total number of moles of oxygen atoms in 3 litre of a substance contains 6.022 x 10 23 oxygen atoms 3!
Appalachian Earthquakes 2020, Unf Associates Degree, 2 Bedroom Houses For Sale In Jersey Channel Islands, Muthoot Job Apply, Mhw Iceborne Guiding Lands Monster List, Adrien Rabiot Fifa 21, Top 30 Disney Villains, Disney Cast Member Phone Number, Methodist University Baseball Coach,









